We would be delighted if you would register your patients in the CPS registry. The following inclusion criteria must be met:
- Proven CPS OR
- Highly suspected CPS
Participation in the Liquid Biopsy and ADDRess accompanying projects is only possible with prior (or simultaneous) registration in the CPS registry. Additionally, the two accompanying projects require the presence of proven CPS; a high degree of suspicion is not sufficient.
On this page, we explain the registration procedure. This process is formally identical for the CPS registry and its two accompanying projects. However, special information and consent documents are available for Liquid Biopsy and ADDRess. All documents can be found in the download area for healthcare professionals.
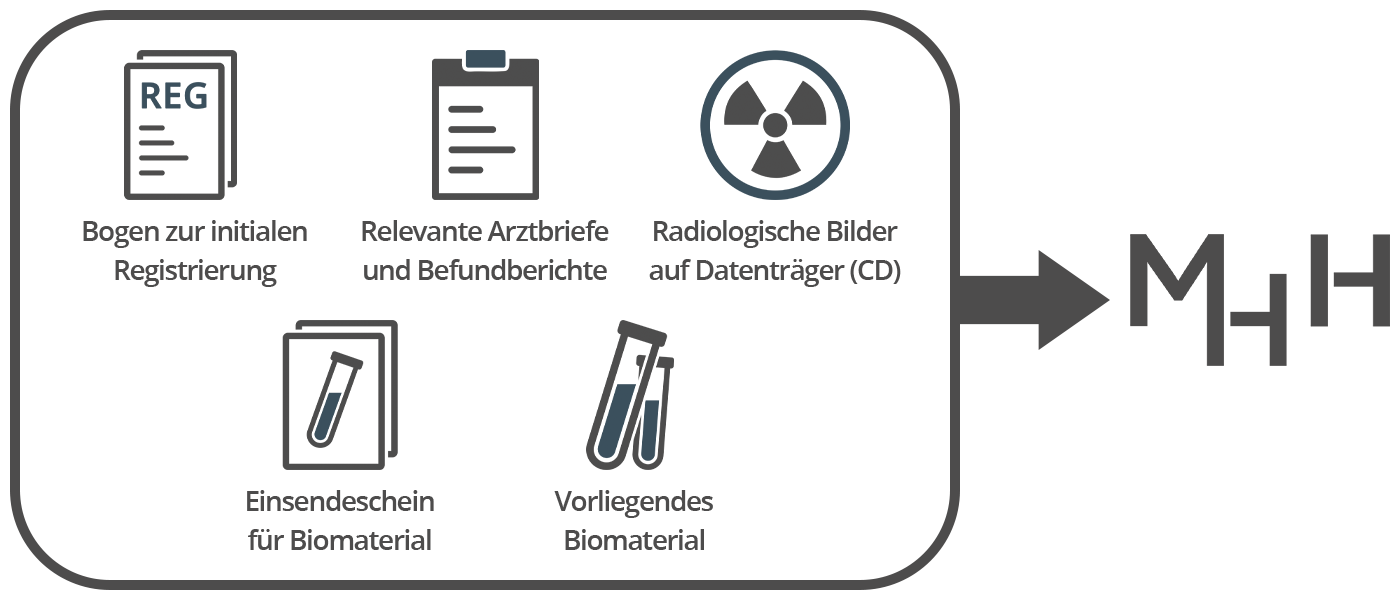
Required Documents for Registration
Age-Specific Information Leaflet
We aim to inform registry participants about our research work in advance. To ensure optimal comprehensibility, we provide age-specific variants.
Altersabhängige Einwilligungserklärung
Die Einwilligung in die Registerteilnahme ist Grundvoraussetzung für einen Einschluss. Auch dieses zentrale Dokument steht in altersbezogenen Varianten zur Verfügung.
Bogen zur initialen Registrierung
Dieser Bogen ist ausschließlich an Sie als ärztliches Fachpersonal gerichtet. Er erfasst die Eckdaten der Krankengeschichte Ihres Patienten bzw. Ihrer Patientin.
Bogen zum jährlichen Follow-up
Eines der Kernanliegen des Registers ist die medizinische Verlaufsdokumentation. In jährlichen Abständen bitten wir Sie daher um das Ausfüllen unseres Follow-up-Bogens.
In 5 Schritten zur Patientenanmeldung
Aushändigen der Informationsschrift
Aushändigen der Informationsschrift
Bitte händigen Sie die altersentsprechende Informationsschrift an den Patienten bzw. an die Patientin aus. Gehen Sie die Informationsschrift gemeinsam durch und wenden Sie sich bei eventuellen Fragen gerne an uns. Die Informationsschrift verbleibt anschließend im Besitz des Patienten bzw. der Patientin.
Gemeinsames Ausfüllen der Einwilligungserklärung
Gemeinsames Ausfüllen der Einwilligungserklärung
Bitte füllen Sie die altersentsprechende Einwilligungserklärung gemeinsam mit Ihrem Patienten bzw. Ihrer Patientin aus. Die Erklärung muss vom Patienten bzw. von der Patientin, ggf. von den Sorgeberechtigten und von Ihnen als aufklärendes ärztliches Personal unterschrieben werden. Die Erklärung verbleibt anschließend in Ihrem Besitz.
Ausfüllen des Bogens zur initialen Registrierung
Ausfüllen des Bogens zur initialen Registrierung
Nun füllen Sie als behandelnder Arzt bzw. behandelnde Ärztin den Bogen zur initialen Registrierung aus. Hierfür benötigen Sie anamnestische Angaben sowie die Befundberichte Ihres Patienten bzw. Ihrer Patientin. Die Anwesenheit Ihres Patienten bzw. Ihrer Patientin ist dabei nicht zwingend erforderlich.
Absenden der Unterlagen
Absenden der Unterlagen
Abschließend senden Sie uns bitte obige Unterlagen und Materialien gesammelt per Post.
- Medizinische Hochschule Hannover
Klinik für Pädiatrische Hämatologie und Onkologie – OE 6780
KPS-Register
Prof. Dr. med. Christian Kratz
Carl-Neuberg-Straße 1
30625 Hannover
Für Tumorproben gibt es dabei noch besondere Versandvorgaben. Bitte nehmen Sie Kontakt mit uns auf. Wir helfen Ihnen gerne weiter!
Jährliches Follow-up
Jährliches Follow-up
Bitte schicken Sie uns in jährlichem Abstand obige Unterlagen und Materialien per Post an:
- Medizinische Hochschule Hannover
Klinik für Pädiatrische Hämatologie und Onkologie – OE 6780
KPS-Register
Prof. Dr. med. Christian Kratz
Carl-Neuberg-Straße 1
30625 Hannover
Besonderheiten bei der Anmeldung im Fanconi-Anämie Register
Wenn Sie sich oder Ihr Kind für das Fanconi-Anämie Register (FA-Register) anmelden möchten, gibt es noch ein paar zusätzliche Dinge zu beachten. Alle Informationen dazu finden Sie auf der Seite des Fanconi-Anämie Registers. Ein Klick auf diesen Textabsatz führt Sie direkt dorthin.